AOAC INTERNATIONAL and Signature Science, LLC, have entered a 3-year Memorandum of Understanding under which Signature Science will produce hemp and cannabis proficiency test samples for AOAC’s new Cannabis/Hemp Proficiency Testing (PT) Program. The partnership was formed to help hemp and cannabis testing laboratories achieve the highest levels of product safety, testing quality, and regulatory compliance through a quality PT program using relevant matrices. With the launch of the program, AOAC became the industry’s only PT provider offering actual >0.3% (low, mid, and high delta-9-THC cannabis) and/or hemp flower as a matrix.
The Cannabis/Hemp PT Program was developed based on feedback from over 500 stakeholders representing more than 200 labs, including state regulatory laboratories, industry laboratories, and state and federal agencies, as well as national and international accrediting bodies, through the AOAC Cannabis Analytical Science Program (CASP).
Cannabis and hemp samples in the program arrive ready to analyze (participants do not have to spike). Labs can choose from different schemes based on their analytical needs. The matrices are dried flower/bud/plant parts. See the list of programs below.
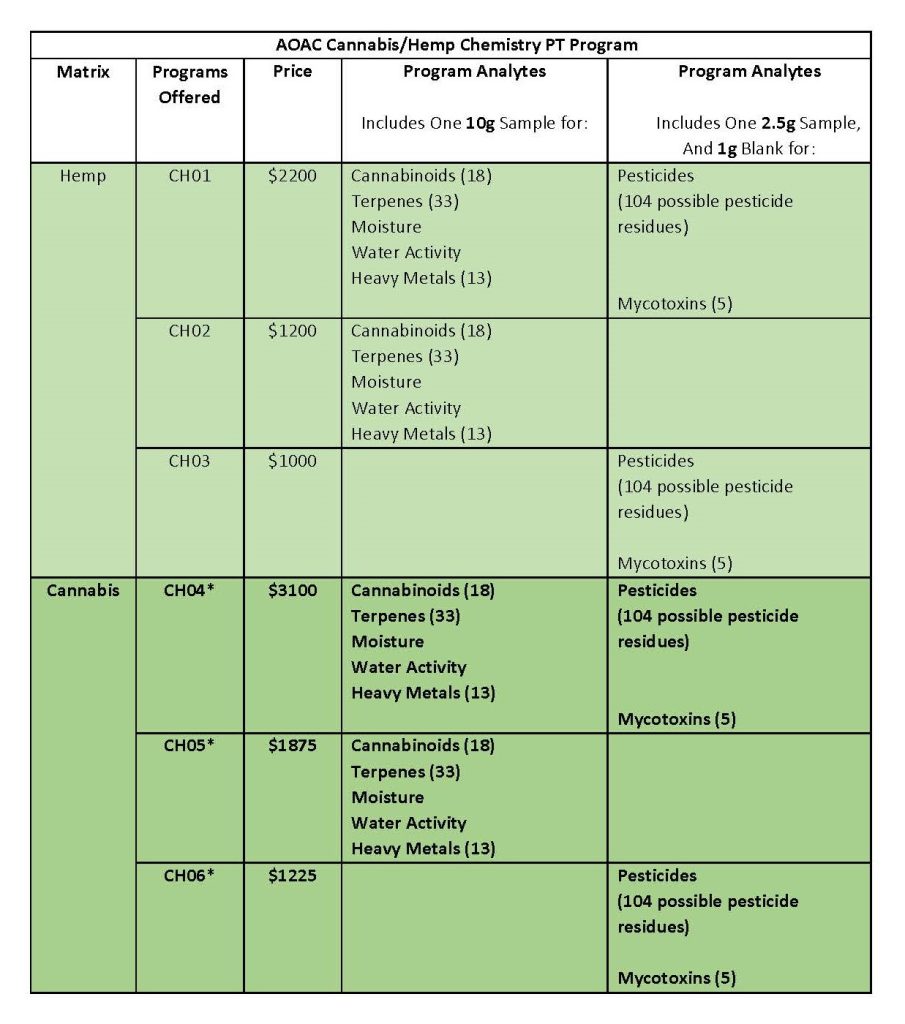
These Hemp and Cannabis PT Programs are currently in the process of being added to the AOAC PT programs scope of accreditation.
*To order CANNABIS from us, an organization must:
For information in obtaining a DEA License and Form 222 please see DEA 222 Form Instructions for Schedule I & II Substances.
AOAC plans to add mycotoxins to the program. In addition, it is anticipated that future programs will offer microbiological samples in both >0.3% THC cannabis and hemp samples. Programs relating to specific matrices, such as gummies and chocolates, are being considered for development.
For questions contact AOAC at [email protected].
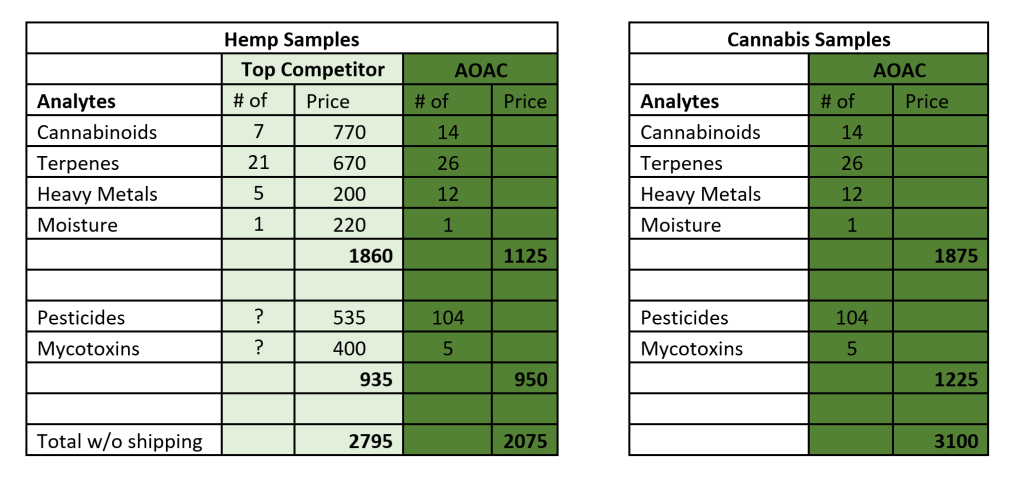
Our samples offer twice the number of cannabinoids, five more terpenes, and seven more heavy metals, than our top competitor. Also, cannabinoids, terpenes, heavy metals, and moisture are all included within one set of our samples, while those analytes are all separate in our competitor’s samples, costing significantly more to order.

Signature Science, LLC is a scientific and technical consulting firm, providing multidisciplinary applied research; technology design and development; and scientific, technical, and operational services to government and industry. Signature Science is an ISO/IEC 17043-accredited PT provider with a DEA- licensed controlled-substance laboratory. The firm holds specialized licenses that allow purchase, storing, handling, and shipping cannabis and hemp test materials to analytical laboratories holding DEA controlled substances licenses across state lines for PT purposes, making SigSci an ideal partner for the AOAC Cannabis/Hemp PT Program. The team at Signature Science developed and validated methods used to create samples for the PT program at its DEA-licensed lab in Austin, Texas, USA. A press release issued by Signature Science states that: “Through an internal research and development effort, Signature Science’s Quality Assurance Chemistry group developed and validated sample preparation, extraction, and analytical testing methods for quantification of cannabinoid potency, terpene profile, and pesticide residues in the hemp and cannabis flower matrix. These methods will be used to create and distribute industry-representative and ISO 17043-compliant samples to testing laboratories throughout the United States.”