Food Colors, Methods & Standards, Current

AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for First Action consideration and possible evaluation through the AOAC Official MethodsTM program. Prospective methods must be able to quantify selected […]
Methods & Standards, Food Colors, Current

AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for First Action consideration and possible evaluation through the AOAC Official MethodsTM program. Prospective methods must be able to quantify selected […]
Methods & Standards, Microbiology
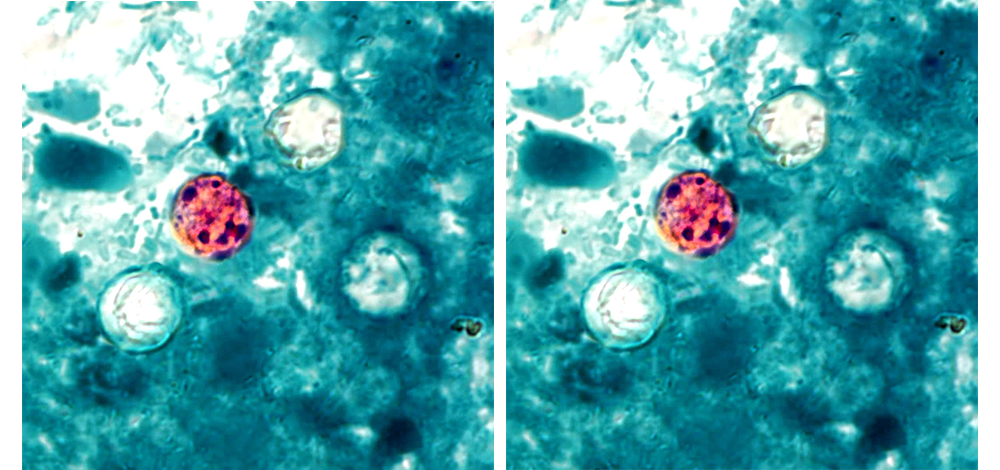
The AOAC Analytical International Methods and Standards (AIMS) program Cyclospora Working Group completed its first draft of Standard Method Performance Requirements (SMPRs®) for Detection, Identification, and Characterization of Cyclospora cayetanensis […]
Food General, Contaminants, Methods & Standards, Current

DEADLINE EXTENDED: Wednesday, April 24 AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for First Action consideration and possible evaluation through the AOAC Official MethodsTM program. Prospective methods must […]
Contaminants, Food General, Methods & Standards, Current

DEADLINE EXTENDED: Wednesday, April 24, 2024 AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for First Action consideration and possible evaluation through the AOAC Official MethodsTM program. Prospective methods […]
Cannabis, Methods & Standards, OMA

The AOAC Cannabis Analytical Science Program (CASP) Working Group recently completed its Standard Method Performance Requirement® (SMPR®) for Detection and/or Enumeration of Listeria monocytogenes in Cannabis Infused Edibles and is […]
Food General, Infant Formula and Adult Nutritionals, Methods & Standards, Journal of AOAC, OMA

DEADLINE: ONGOING UNTIL FURTHER NOTICE Determination of α-Carotene in infant formula, adult/pediatric formula and select milk and milk products AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for consideration and […]
Food General, Infant Formula and Adult Nutritionals, Journal of AOAC, Methods & Standards, Current, OMA

DEADLINE: ONGOING UNTIL FURTHER NOTICE Determination of Phospholipids in infant formula, adult/pediatric formula and select milk and milk products AOAC INTERNATIONAL (AOAC) invites method developers to submit methods for consideration and […]
Allergens, Food General, Methods & Standards

Draft AOAC Food Allergen Immunoassay Validation Guidance Document (September 2023) Open for 30-Day Public Comment Period September 13, 2023: AOAC INTERNATIONAL (AOAC) announces the availability of the draft Food Allergen […]
Infant Formula and Adult Nutritionals, Methods & Standards

Open for 30-Day Public Comment Period September 13, 2023: AOAC INTERNATIONAL (AOAC) announces the availability of draft Standard Method Performance Requirements (SMPR®) for Vanillin, Ethyl Vanillin, Methyl Vanillin, and Coumarin […]
Contaminants, Food General, Methods & Standards

The AOAC PFAS Working Group recently completed its review and revision of the SMPR for Per- and Polyfluoroalkyl Substances (PFAS) in produce, beverages, dairy products, eggs, seafood, meat products, and […]
Dietary Supplements, Food General

The AOAC Botanical Ingredients and Dietary Supplement Integrity (BIDSI) program’s Pyrrolizidine Alkaloids Working Group recently completed its review and revision of the SMPR for Pyrrolizidine Alkaloids in teas, herbal infusions, […]
Food Colors, Contaminants, Methods & Standards
DEADLINE: Friday, July 28, 2023 The AOAC Residual Solvents Subgroup within the Color Additives from Natural Colors Working Group recently completed its review and revision of the SMPR for Selected Residual […]
Contaminants, Food General, Methods & Standards, New Initiatives
DEADLINE: July 7, 2023 The AOAC Heavy Metals Working Group recently completed its review and revision of the SMPR for the Determination of Trace Elemental Contaminants in Food and Beverages and […]
Cannabis

AOAC CASP Pesticide Method Think Tank working group is refocusing to create a best practices document to develop and validate methods for pesticide residue analysis in cannabis. Efforts will be […]
Annual Meeting, Microbiology, Agricultural Materials, Cannabis, Contaminants, Food Colors, Food General, Dietary Supplements

The 2024 AOAC Call for Poster Abstracts will be open from Wednesday, May 1, 2024 to Wednesday, May 29, 2024. The Call for Poster Abstracts will close on Wednesday, May […]
Food General, Journal of AOAC, Contaminants

Call for Papers: Methods for detection, quantitation, removal and/or mitigation of chemicals of concern from food matrices and packaging materials Guest Editor: Greg Curtzwiler, Ph.D. The Journal of AOAC INTERNATIONAL […]
Membership

November 18, 1932-March 15, 2024 George Webster Latimer, Jr., a Fellow of AOAC (1997) and a long-time member, passed away on March 15, 2024. He was 92 years old. Latimer […]